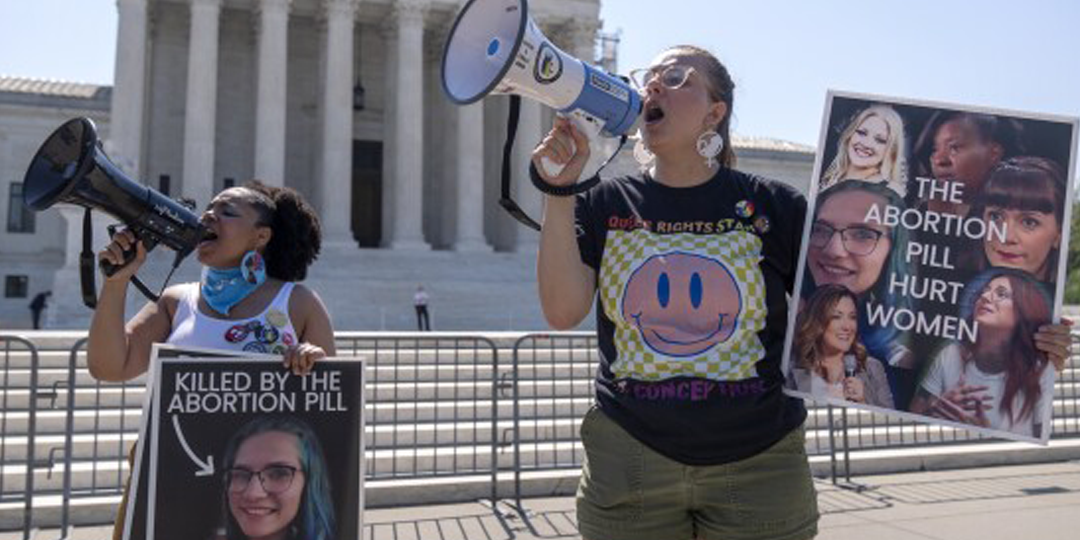The U.S. Supreme Court on Thursday rejected a lawsuit challenging the Food and Drug Administration’s (FDA) approach to regulating the abortion pill mifepristone, allowing the drug to continue to be provided by mail without an in-person doctor’s visit. In a unanimous decision, written by Justice Brett Kavanaugh, the Court held that the plaintiffs did not have legal standing to challenge the FDA’s actions.
At issue was a challenge by pro-life medical organizations to the FDA’s long-standing approval of mifepristone. The group argued that the FDA did not adequately review safety risks in its initial approval. Plaintiffs also challenged recent changes by the FDA to loosen restrictions on the drug, allowing patients to receive it via telemedicine and through the mail. The Court ruled that the plaintiffs did not demonstrate how they would be harmed by the FDA’s actions.
While the decision was a setback for pro-lifers, the Court did not rule on the merits of the FDA’s approval and regulation of mifepristone, leaving the door open for future challenges.